エンダース論文(1954)英語
原著:「Propagation in Tissue Cultures of Cytopathogenic Agents from Patients with Measles」(PDF)
邦訳:「麻疹患者からの細胞変性病原体の組織培養による増殖」(PDF)
著 者:J. Enders, T. Peebles
掲載年:1954年6月1日
出版元:Medicine, Biology
掲載誌:Proceedings of the Society for Experimental Biology and Medicine
Propagation in Tissue Cultures of Cytopathogenic Agents from Patients with Measles
JOHN F. ENDERS AND THOMAS C. PEEBLE
(With the assistance of Yinette Chang and Ann Holloway.)
From the Researah Division of Infectious Diseases, Children's Medical Center, Boston, Mass. and Departments of
Bacteriology and Immunology and of Pediatrics, Harvard Medical School
Numerous attempts have been made in the past to propagate the agent of measles in lower animals, in chick embryos and in tissue cultures(1-3). The results of different investigators were often at variance or directly contradictory. It has been made reasonably clear, however, that monkeys, especially M. mulatta, are moderately susceptible to experimental inoculation (3). Furthermore the researches of Rake, Shaffer and their collaborators have provided evidence suggesting that the agent which passed through bacteria-retaining filters could be maintained indefinitely in serial passages in the developing chick embryo(4, 5). These workers (5) also confirmed the earlier observations of Plotz(6) who apparently had succeeded in growing the agent in a modified suspended cell culture of chick embryonic tissues. Egg passage in the hands of Shaffer and his coworkers(7,8) regularly appeared to alter the pathogenicity of the agent for man as indicated by the development of a mild and much modified disease following the inoculation of egg adapted materials into susceptible children. In certain cases this modified disease seemed to be followed by resistance to measles as indicated by the results of subsequent natural or artificial exposure to the virulent form of the agent(9). Since 1943 when the last of the communications by Rake and his collaborators appeared, no important progress has been made in the study of the etiology of measles. This fact may in large part be attributed to the lack of a convenient laboratory method for the demonstration of the presence of the agent which induced no recognizable changes in eggs or cultures of chick tissues. Moreover, repeated attempts by Shaffer(10) to demonstrate a serologic reaction, such as complement fixation, using materials from the infected chick embryo failed. Accordingly, the only available technics have consisted in the inoculation of man or the monkey. The former is obviously impractical as routine and the latter tedious, expensive and frequently inconclusive because of variation in individual susceptibility.
With these considerations in mind we have recently attempted to cultivate the agent of measles in cultures of human and
monkey cells employing procedures applied successfully to the propagation of the poliomyelitis viruses(11-13). In blood and throat
washings of typical cases of measles agents have been demonstrated that can be maintained in serial passage in tissue cultures and which
induce distinctive cytopathic changes in renal epithelial cells. A certain amount of evidence has been accumulated
indicating that antibodies specific for these agents develop during
the course of the disease. It is our purpose to describe here these observations in a preliminary manner. Additional
evidence for the relationship of these agents to measles will be sought in future investigations.
TABLE I.
Cytopathogenic Agents Isolated in Tissue Cultures from Throat Washings and Blood of 5 Measles Cases.

Materials and methods.
Collection of specimens.
Throat washings, venous blood and feces were obtained from 7 patients as early as possible after a clinical diagnosis of measles
was established. In 5 instances the time at which specimens were collected in relation to the onset
of exanthem is given in the case histories described below or in Table I. When capable, patients were
asked to gargle with 10-15 ml of sterile neutralized fat-free milk.
Certain specimens from the throats of younger children were obtained by cotton swab previously moistened in milk. After swabbing the throat the swab was
immersed in 2 ml of milk. Penicillin, 100 u/ml, and streptomycin, 50 mg/ml, were added to all throat specimens which were then centrifuged at 5450 rpm
for about one hour. Supernatant fluid and sediment resuspended in a small volume of milk were used as separate inocula in different
experiments in amounts varying from 0.5 ml to 3.0 ml. About 10 ml of blood immediately after withdrawal were placed in tubes containing 2 ml
of 0.05% solution of heparin. As inocula for tissue cultures amounts varying from 0.5 ml to 2.0 ml of the whole blood were employed. After
addition of antibiotics as described above 10% fecal suspensions were prepared by grinding the material in bovine amniotic fluid medium. The
suspensions were then centrifuged at 5450 rpm for about one hour and the supernatant fluids used as
inocula, in amounts varying from 0.1 ml to 3 ml. All specimens were refrigerated in water and ice
or maintained in the cold at about 5°C from the time of collection until they were added to the cultures. The maximum time
that lapsed between collection of specimens and inoculation was 3 1/2 hours.
Tissue culture technics.
In the initial isolation attempts roller tube cultures (11, 12) of human kidney, human embryonic lung, human embryonic intestine, human uterus and rhesus monkey testis were employed. Subsequent passages of the agents isolated were later attempted in human kidney, human embryonic skin and muscle, human foreskin, human uterus, rhesus monkey kidney and embryonic chick tissue. Stationary cultures prepared according to the technic of Youngner(13) with trypsinized human and rhesus monkey kidney were later employed for isolation of agents and their passage. The culture medium consisted of bovine amniotic fluid (90%), beef embryo extract (5%), horse serum (5 %) , antibiotics, and phenol red as an indicator of cell metabolism (12). Soybean trypsin inhibitor was added to this medium unless it was used for the cultivation of human and monkey kidney (11). Fluids were usually changed at intervals of 4-5 days. For histological examination the cell growth after fixation in 10% formalin was embedded in collodion, dehydrated and stained with hematoxylin and eosin.
Manner of passage in tissue culture.
Serial passage of the various strains (Table I) was accomplished as routine by removal of the culture medium between the 4th and the 16th day after inoculation and immediate transfer of 0.1 ml to each of a number of fresh cultures. Successful passage of the agent with fluids that had been previously centrifuged at 2500 rpm to remove cellular elements has also been repeatedly demonstrated. Larger inocula (up to 1.0 ml) were often used during the initial experiments before the resistance of the agent to storage at various temperatures had been determined.
Virus neutralization and complement fixation tests.
The procedures employed are described subsequently in the text.
Description of cases from which materials were obtained.
During an outbreak of measles at a private boarding school for boys in Southboro, Mass., isolations were attempted from throat washings and stools of 4 patients and blood of 3 of the same patients. The latter are designated as Cases 1, 2 and 3. Throat washings of 2 siblings in a small epidemic of measles in Wellesley, Mass., (Cases 4 and 5) and blood from 2 cases of measles admitted to the Boston City Hospital during an epidemic in Boston (Cases 6 and 7) are under investigation at the present time. Details of these typical cases are omitted for the sake of brevity.
Case I :
D.R., age 11, was in contact with .a "probable" case of measles 10 days before symptoms commenced on 1/20/54. The latter consisted of signs of an upper respiratory infection including sore throat and fever to 101°F. He soon developed conjunctivitis and a bad cough. These symptoms became aggravated and on 1/24 in the morning there was a suggestion of a rash on his face. Temperature at 4:30 p.m. was 105.5°F. Koplik's spots were noted on the buccal mucosa by the school physician the next morning. On 1/25 at 1 p.m. he was seen by TCP with findings of temperature 98° p.o., mild to moderate conjunctivitis, moderate generalized adenopathy and characteristic blotchy maculopapular rash in full bloom, extending to involve even the palms and soles. No Koplik's spots were seen at this time. Specimens of throat washings, blood and stool were collected at 1:30 p.m. on 1/25.
Case 2:
H.J., age 13, with no known contact other than Case 1, developed signs of an upper respiratory infection on 1/26/54. He
complained of sore throat and cough the following day and of photophobia on 1/28.
At this time the infirmary nurse noticed a questionable trace of a rash on his forehead. He was seen on
1/29 by TCP with findings of temperature 101° p.o., and maculopapular rash developing over the face, ildly over the chest
and abdomen, minimally on the upper extremities and none on the lower extremities. He had moderate
conjunctivitis, cough and' Koplik's spots on the buccal mucosa. Specimens of throat washings, blood and stool were
obtained at 11 a.m. on 1/29.
Case 3:
D.E., age 13, a close contact of both Cases 1 and 2 during their prodromal stages, experienced a
gastrointestinal upset on 2/8/54 with cramps and nausea. Temperature was 99.6°F. Faint rash was first noted on his face
in the late evening of 2/9, and he was seen by TCP at 12 noon on 2/10 with findings of typical rash over the face
with minimal extension to the chest, abdomen and back. Koplik's spots were present on the buccal mucosa. Cough
and conjunctivitis were mild. His temperature was 102° p.o.
Specimens of throat washings, blood and stool were obtained at this time. His temperature was 103.6°F with rash
in full bloom in the afternoon. On the following morning his temperature was 104°F.
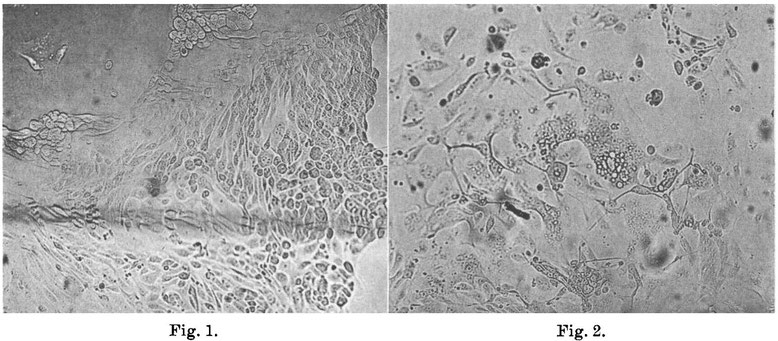
FIG. 1.
Outgrowth of normal huimn kidney cells in a n uninoculated roller tube culture (X 130). Control for cultures shown in Fig. 2 and 3.
FIG. 2.
Area of syncytial giant cells with small cytoplasmic vacuoles. Note many nucleoli and faint nuclear outlines. 9th day after inoculation; 7th passage, agent from Case 3 blood (X 130) .
Experimental.
Cytopathic changes induced by agents isolated from cases of measles.
The first of 8 agents obtained from blood or throat washings of measles cases and exhibiting comparable properties was isolated in cultures of human kidney tissue following addition of the blood of Case 3. In each of the 3 cultures that were inoculated cytopathic changes were observed on the 7th day. Since these changes presented a characteristic appearance not heretofore associated definitely with a virus they have provided the means for the further investigation of this agent as well as others that have been recently isolated. Accordingly, here at the beginning these changes will be described in detail. Observation of fresh preparations under low magnification (80X) revealed within the sheet-like outgrowth of renal epithelial cells discrete areas of varying size and shape in which the cell boundaries were obliterated and the nuclei often difficult to visualize. Within these areas, which may be described as non-refractile "glassy" plaques, large and small vacuoles were often numerous lending them a foamy or lace-like quality. The number and size of the vacuoles increased as incubation was continued. On careful examination of these areas many small, slightly refractile bodies were seen that resembled nucleoli within nuclei whose outlines could often be distinguished only with difficulty. The total effect thus suggested the presence of large vacuolated giant cells. After further cultivation the extent of the areas initially present was slowly extended or was enlarged by coalescence with neighboring plaques while others developed elsewhere. In addition to the formation of vacuoles degenerative changes gradually appeared within the affected areas suggesting coagulation necrosis. At the end of three weeks most of the epithelial cells appeared to be involved, yet here and there small aggregates of normal cells remained. These seemed, however, to be composed mainly of spindle-shaped cells. Reference to Fig. 1, 2 and 3 will aid in the visualization of these changes as they are manifest in the natural state. In contrast to the appearance of the normal cell outgrowth shown in Fig. 1 the smooth confluent area of affected cells stands out clearly in Fig. 2. While a slight degree of vacuolization is evident in this figure, it is extensive in Fig. 3 especially along the margin of cell growth where it is first apt to become apparent.
The interpretation that has just been presented of the changes observed in fresh preparations was supported by examination of fixed and stained materials. Under these conditions the glassy areas were clearly revealed as collections of nuclei surrounded by a common cytoplasmic matrix. As many as 40 to 100 nuclei were counted in such syncytial formations. Often the limits of the encompassing cytoplasm were sharply defined thus contributing to the impression that evelopment of true giant cells has occurred in vitro. Whether or not this is actually the case, the phenomenon is of much interest in view of the constant presence of giant cells in lymphoid tissues during the early stages of measles in man(14,15) .
Examination of stained materials also revealed significant changes within the nuclei of the giant cells that were not
visible in fresh preparations. These consisted in a redistribution of the chromatin which ultimately assumed a marginal
position where it formed a dense ring or crescent that stairied intensely with the basic dye. Concomitantly the central portion of the
nucleus came to be occupied by an apparently homogeneous substance, acidophilic in character, that approximated closely to the
chromatin ring. Since in these and other preparations that have been examined subsequently no clear unstained zone has been
observed between the chromatin and this acidophilic mass, it cannot be asserted that the latter represents an intranuclear
inclusion body of the type characteristically associated with viral infections. Nevertheless, as far as can now
be determined, its presence along with the margination of the chromatin affords a useful criterion of infection
for the agents under study. It should be emphasized, however, that the changes as just depicted are encountered in cultures
that have been incubated for relatively prolonged periods (e.g. 14-21 days). When the interval between inoculation of the
agent and examination of the stained cells (e.g. 4 days) is shorter, margination of the chromatin may be
incomplete or inapparent and the acidophilic substance may only be seen in small rounded masses
distributed here and there amid nuclear materials that approximate the normal arrangement.
Fig. 4, 5, 6 and 7 illustrate well-developed nuclear changes and also the general similarity of the affected areas to the giant
cells encountered in lesions associated with measles. Of particular interest in this latter connection are the basophilic “pseudoprotozoal” bodies
that Bonenfant (16) has recently described in the mucosa and lymphoid follicles in cases of this disease. These bodies were usually surrounded by
an acidophilic homogeneous substance. As described and pictured these bodies with their matrix strikingly resemble the giant cells in tissue
cultures that exhibit well-developed nuclear changes.

FIG. 3.
Lace-like network of vacuoles at tissue margin from the same culture ( X 130).
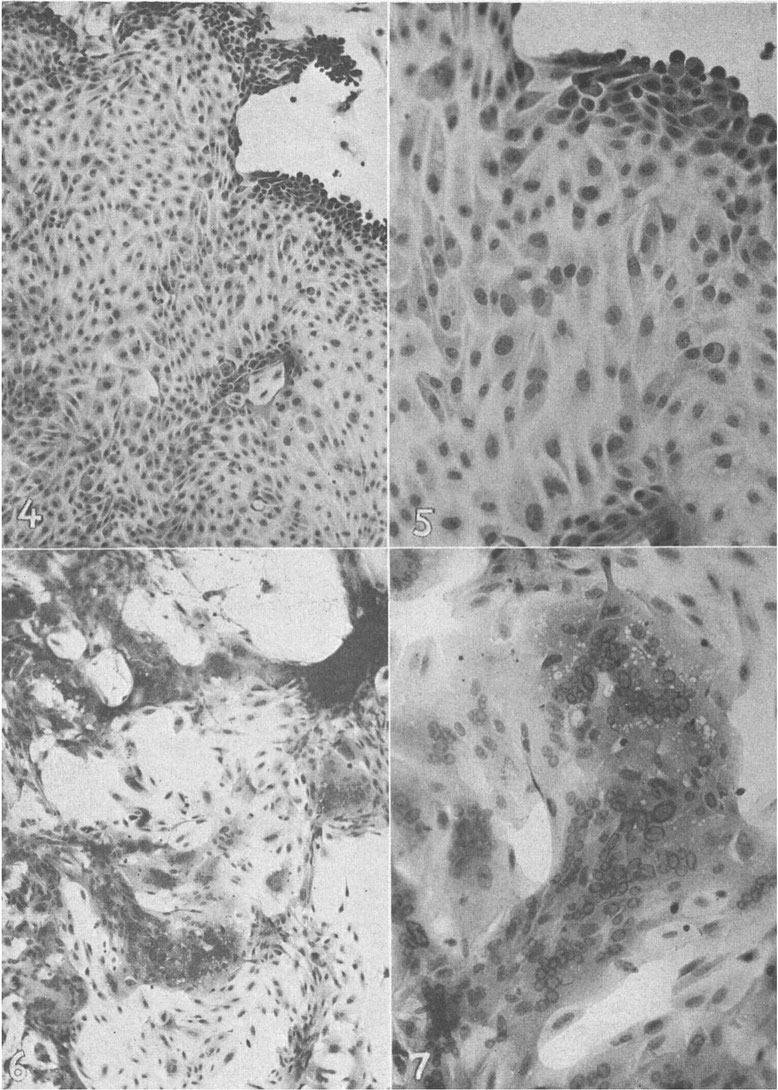
FIG. 4.
Outgrowth of normal human renal cells fixed and stained with hematoxylin and eosin. Control for cultures shown in Fig. 5-7 (×110).
FIG. 5.
A portion of the cell outgrowth shown in Fig. 4 more highly magnified. Hematoxylin and eosin stain (×300).
FIG. 6.
Outgrowth of human renal cells showing giant cell formation and nuclear changes 20 days after inoculation with 2 ml blood from Case 3. Hematoxylin and eosin stain (×110).
FIG. 7.
A portion of cell outgrowth shown in Fig. 6 more highly magnified. Hematoxylin and eosin stain ( X 300).
Some biologic properties of the agents isolated from measles.
Certain of the biologic properties of the agents isolated from patients with measles have been definitely determined, others in a preliminary or tentative manner. In several instances these properties have been studied only in respect to the strain first isolated from the blood of Case 3. Since, however, the other strains, as far as they have been examined, behave in a similar manner it is probable that all of them, when thoroughly studied, will exhibit the same general characteristics.
A) Source of virus.
As repeatedly stated, agents have been recovered from both blood and throat washings. In three cases yielding viruses from one or another of these sources fecal suspensions have likewise been examined by the tissue culture technic. In none was evidence for the presence of an agent obtained. Further examinations of fecal specimens are necessary before it can be stated whether or not the virus is present in the intestinal excreta.
B ) Cytopathogenic range.
Monkey kidney is the only other tissue employed that has yielded a growth of cells in which the characteristic changes described above have been definitely observed following inoculation of virus. In cultures consisting largely of monkey renal epithelial cells as prepared by Youngner’s modification of Dulbecco’s technic (13) cytopathic changes have been regularly observed which resemble closely those produced by these agents in human renal cells as seen in both fresh and stained preparations. These effects followed the addition of blood or throat washings from cases of measles as well as infected tissue culture fluids derived from previous passages. Monkey kidney cultures may, therefore, be applied to the study of these agents in the same manner as cultures of human kidney. In so doing, however, it must be borne in mind that cytopathic effects which superficially resemble those resulting from infection by the measles agents may possibly be induced by other viral agents present in the monkey kidney tissue (c f. last paragraph under G) or by unknown factors. In a few cultures of human prepucial tissue inoculated with one of the measles agents changes resembling those seen in renal cells were noted in the epithelial outgrowth about certain fragments. Additional observations, however, will be required before it can be confidently asserted that dermal epithelial cells are specifically attacked by these viruses. In a single experiment no cytopathic manifestations were seen during a period of 31 days following inoculation of infected tissue culture fluid into cultures of human embryonic skin and muscle, human uterine tissue or embryonic chick tissue. Tests for the presence of complement fixing antigen in the fluids removed from the cultures on the 31st day were negative. These serologic results suggest that growth of the virus did not occur, since, as will be shown subsequently, the antigen appears to develop regularly after several days in cultures of renal tissue infected with the virus.
C) Failure to induce demonstrable changes in mice or chick embryos.
Two litters of suckling white mice (1-day-old) were inoculated with infective tissue culture fluid by both the intraperitoneal and intracerebral routes. The animals remained well during an observation period of 21 days. Employing the same material as inoculum 0.1 ml amounts were introduced into the amniotic sac of 7-day embryonated hen’s eggs. After 7 days incubation at 36°C the amniotic fluid and membranes were harvested, ground with alundum and centrifuged at 1500 rpm. The supernatant fluid was used for a second egg passage which was carried out in the same manner. Whereas inoculation of 0.1 ml aliquots of the first egg passage material into cultures of monkey renal epithelium was followed by characteristic cytopathic changes on the 8th day, the addition of 0.5 ml of second egg passage material to such cultures failed to produce this effect. No complement fixing antigen was detected in the materials from the egg passages. Although these results suggest that the virus is not readily adapted to growth in the chick embryo, it is evident that much further investigation will be required to determine the degree of susceptibility of this host.
D) Serial passages.
Serial passages of several of the strains have been carried out with out difficulty in cultures of human or monkey renal cells. Employing 0.1 ml of the fluid phase as inoculum, 10 passages of the first strain isolated have so far been accomplished in human cells of this type. With other strains fewer passages have been completed as indicated in Table I.
E) Assay of infectivity.
As yet only one attempt has been made to measure infectivity of virus propagated in tissue culture.
In this case fluids and cells were removed from 15 cultures of human kidney tissue on the 6th day
after inoculation of fluid from the 4th tissue culture passage of the agent from Case 3. These materials were pooled and the cells were
ground with alundum in the presence of the fluid. After centrifugation for 15 minutes at 2500 rpm the supernatant fluid was titrated for infectivity in
cultures of human kidney tissues.
For this purpose 3 cultures were each inoculated with 0.1 ml of the suspension diluted by a factor of 10.
The endpoint of viral activity as indicated by the highest dilution causing cytopathic changes was about 10-2.5. This low titer was somewhat
unexpected in view of the fact, as will be shown hereafter, that tissue culture fluids contain sufficient antigen to fix
complement in the presence of convalescent measles serum. Without more experimental data, however, it cannot be assumed that maximal infectivity
titers lie within this range.
F) Seutralization of cytopathogenicity by convalescent measles sera.
That the cytopathogenic capacity of at least one strain of the agents associated with measles is inhibited by serum
factors developing during the course of the disease has been demonstrated in two experiments.
Employing 100 ID50 of the viral suspension mentioned in the previous paragraph neutralization tests were carried out in cultures
of monkey renal epithelial cells. Sera taken during the acute and convalescent stages from two of the cases occurring at the
boys’ school were stored at -15°C and inactivated at 56°C for 30 minutes before they were diluted and used in the test. As
diluent bovine amniotic fluid was employed. Dilutions of serum and virus were mixed and kept at 5°C for one hour when 0.1
ml of each mixture was added to each of three tissue cultures. In both tests the cultures were examined every
day or every 2 days and the final readings were recorded on the 10th day. The results are summarized in Table 11. They indicate that significant increases in
substances occurred in the serum of both patients that neutralized the cytopathogenicity of the agent isolated from the blood of a
third patient. In considering these results it is pertinent to recall that agents with similar characteristics have been isolated from the
two patients whose convalescent sera were shown to possess virusneutralizing capacity.
TSBLE II.
Neutralization of Cytopathogenic Effect of Virus from Measles Case 3 by Convalescent Sera from Two Other Cases of Measles. *Readings taken on 10th day after addition of virus.

G ) Production of complement fixing antigen in tissue cultures.
Since it has been shown (17) that in cultures of poliomyelitis viruses antigens capable of fixing complement in the presence of specific antibodies appear in the fluid phase, tests were carried out to determine whether or not the fluids removed from cultures exhibiting cytopathic changes induced by the measles agents might behave in a similar manner. To this end the drop method of Fulton and Dumbell(18) as modified by Svedmyr, Enders and Holloway(17) was employed. As antigens crude undiluted fluids have been used. These were derived from human or monkey kidney cultures inoculated with strains isolated from either blood or throat washings of Cases 2, 3 and 4. The fluids from several cultures were collected at various intervals after inoculation of the virus, pooled, centrifuged at 1500 rpm for 5 minutes and stored at either 5°C or -16°C. Immediately before use the fluid was heated at 56°C for 30 minutes to remove any anticomplementary activity that might be present. As control antigens, fluids were taken from uninoculated cultures maintained under the same conditions as well as fluids from cultures of the agent producing changes superficially similar to those caused by the measles agents and which are mentioned below. These materials failed to fix complement with any of the sera that have been examined. Acute and convalescent phase measles sera were inactivated at 56°C and serial dilutions prepared as in complement fixation tests for poliomyelitis antibody. The results of tests that have so far been completed indicate clearly that antibodies develop during the course of measles capable of reacting with an antigen that appears in the culture fluid after the 3rd to the 7th day following inoculation of the virus. From representative data presented in Table III it is evident that the antibody may emerge at least as early as the 7th day following the appearance of the rash and continues to persist for at least 2 months in fairly high titer. By this time, however, there is some indication that the maximal concentration has been previously attained. It is noteworthy in respect to the possible etiologic relationship of these agents to measles that antibodies appeared in the blood of Cases 6 and 7 which fixed complement with the antigens from Cases 2 and 3. The latter occurred in a widely separated area and at an earlier time. A few tests have been carried out with measles antigen on sera from 3 adults giving a history of measles during childhood. Serum titers of 1:2, 1:8 and >1:16 were recorded.
TSBLE III.
Results of Complement Fixation Tests on Measles Sera Employing as Antigens Fluids from Tissue Cultures Infected with Agents Isolated from Cases of Measles.
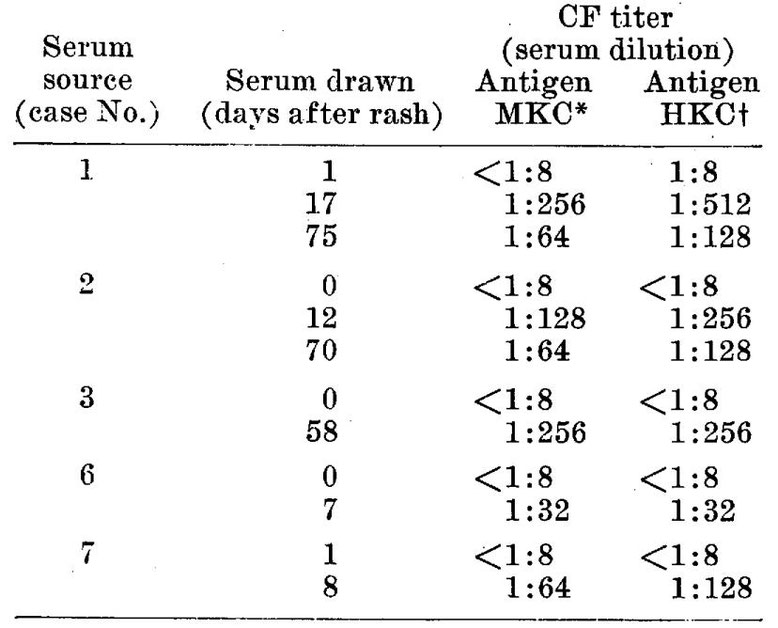
* MKC = Monkey kidney tissue culture fluid infected with agent from blood of case 2.
† HKC = Human kidney tissue culture fluid infected with agent from blood of case 3.
Filterability and resistance to physical agents.
Filtration.
A portion of the pool of virus with infectivity titer of l0-2.5 (cf. last paragraph under E)was diluted 1:5 in
beef infusion broth and passed through a sintered glass filter under a pressure of 45 mm Hg.
Time of filtration was 10 minutes. The capacity of the filter to retain Serratia marcescens was then demonstrated. The
viral filtrate was shown to be free of bacteria by addition to infusion and thioglycollate broth and blood agar media. Five cultures of
monkey renal cells were inoculated with the filtrate (0.5-1 ml). Characteristic cytopathic changes were subsequently noted in all.
Thermal stability.
The cytopathogenicity of one strain was destroyed by heating at 65° for 30 minutes. The infectivity of heparinized blood or throat washings in milk as tested in tissue culture was preserved for at least 3 1/2 hours by refrigeration at 1°C to 5°C. Agents present in tissue culture fluids remained infective after 38 days at -15°C and after at least 35 days at about -50°C to -60°C.
Other agents isolated during this study.
Two agents have been isolated while the present work was in progress that appear unrelated to those we have just described. The first was recovered from the throat washings of a typical case of measles occurring in the boys' school. Its wide cytopathogenic range, the character of the cytopathic changes induced and the fact that its infectivity for tissue cultures was neutralized by herpes simplex immune rabbit serum served to define its nature. A second agent was obtained from an uninoculated culture of monkey kidney cells. The cytopathic changes it induced in the unstained preparations could not be distinguished with confidence from the viruses isolated from measles. But, when the cells from infected cultures were fixed and stained, their effect could be easily distinguished since the internuclear changes typical of the measles agents were not observed. Moreover, as we have already indicated, fluids from cultures infected with the agent failed to fix complement in the presence of convalescent measles serum. Obviously the possibility of encountering such agents in studies with measles should be constantly kept in mind.
Discussion.
Of the numerous experiments that have been reported in the past describing the successful isolation of the etiologic agent of measles only those in which
monkeys were employed as the experimental animal have been consistently confirmed by other workers. Great caution
should therefore be exercised in the interpretation of any new claims that the virus has been propagated in
other hosts or systems. Accordingly, the results that are summarized here must be subjected
to the most critical analysis.
The following facts tend to support the hypothesis that the viruses we have described are responsible for the disease. Experimentally transmissible
agents exhibiting a similar and characteristic cytopathogenic effect in cultures of human or simian epithelial cells have been isolated from either the blood or throat
washings derived from 5 of 7 typical cases of measles during the early acute phase. An agent was
demonstrated in the blood of 4 of the 5 cases from which specimens were obtained and examined
by the tissue culture method. These findings would seem to be of especial significance since it is unlikely that viruses
unrelated to measles would be regularly present in the circulating blood of these individuals some of whom were geographically widely
separated.
The pathologic changes induced by the agents in epithelial cells in tissue culture resemble, at least
superficially, those found in certain tissues during the acute stage of measles. While there
is no ground for concluding that the factors in vivo are the same as those which underlie the formation of giant cells and
the nuclear disturbances in vitro, the appearance of these phenomena in cultured cells is consistent with the properties that a
priori might be associated with the virus of measles.
The emergence of antibodies during the course of the disease capable of suppressing the cytopathogenic effect
and of fixing complement in the presence of infected tissue culture fluids affords further evidence for the close
association of the agents with measles. Obviously additional data to be derived from tests with sera from a large
number of cases of measles as well as other infectious diseases, especially the common exanthemata, are desirable in order to eliminate any remaining doubt concerning the
specificity of these serologic reactions. The accumulation of such data is now in progress.
Although we have thus already obtained considerable indirect evidence supporting the etiologic role of this group of agents in
measles, 2 experiments essential in the establishment of this relationship remain to be carried out. These will consist in the
production of measles in the monkey and in man with tissue culture materials after a number of passages in vitro sufficient to eliminate any
virus introduced in the original inoculum. The recovery of the virus from the experimental disease in these hosts should then be
accomplished.
Conclusion.
The findings just summarized support the presumption that this group of agents is composed of epresentatives of the viral species responsible for measles.
Summary.
Eight agents exhibiting the properties of viruses have been isolated in cultures of human or simian renal cells from the blood or throat washings of five cases of typical measles. Multiplication of the agents in vitro is accompanied by characteristic changes in the cells. Primarily these changes consist in the formation of syncytial giant cells wherein the chromatin assumes a marginal position and is replaced centrally by an acidophilic substance of unknown nature. The cytopathogenic effect of at least one of the agents is inhibited by convalescent phase measles sera from other patients with measles. Antigen appears during cultivation in vitro of the measles agents that reacts specifically in complement fixation tests with convalescent phase measles sera.
1. Enders, J. F., Virus and Rickettsial Diseases, Harvard University Press, Cambridge, Mass., 1940, pp237-267.
2. Van Raooyen, C. E., and Bodes, A. J., Virus Diseases of Man. Thomas Nelson and Sons, New
York, 1948, pp226-228.
3. Rake, G., Measles in Viral and Rickettsial Infections of Man, ed. T . M. Rivers, J. B. Lippincott Co., Philadelphia, 1952, pp480-481.
4. Rake, G., and Shaffer, M. F., J. Immunol., 1940, v38, 177.
5. Rake, G., Shaffer, M. F., and Jones, H. P., J. Inf. Dis., 1941, v69, 65.
6. Plotz, H., Bull. Acad. Med., 3rd Ser., 1938, v119, 598.
7. Shaffer, M. F., Rake, G., Sitokes, J., Jr., and O’Neil, G. C., J. Immund., 1941, v41, 241.
8. Stokes, J., Jr., O’Neil, G. C., Shaffer, M. F., Rake, G., and Mark, E. P., J. Pediat., 1943, v22, 1.
9. Maris, E. P., Rake, G., Stokes, J., Jr., Shaffer, M. F., and O’Neil, G. C., ibid., 1943, v22, 17.
10. Shaffer, M. F., Personal communication to JFE.
11. Rsobbins, F. C., Weller, T. H., and Enders, J. F., J. Immunol., 1952, v69, 673.
12. Enders, J. F., hoe. Soc. Em. BIOL. AND Mm., 1953, v82, 100.
13. Youngner, J. S., ibid., 1954, VSS, 2012.
14. Warthin, A. S , Arch. Path., 1931, v l l , 864.
15. Mulligan, R. M., ibid., 1944, v37, 61.
16. Bonenfant, J. L., Arch. Fran. de Pediatrie, 1952, v9, 497.
17. Svedmyr, A., Enders, J. F., and Holloway, A., Proc. Soc. Exp. BIOL. AND MED., 1952, v79, 296.
18. Fulton, F. and Dumbell, K. R., J. Gen. Microbiol., 1949, v3, 97.
Received May 16, 1954. P.S.E.B.M., 1954, v86.
